| Home | |
| Faculty | |
| Graduate Program | |
| Events and Seminars | |
| Resources | |
| Contact Us | |
|
F A C U L T Y P R O F I L E
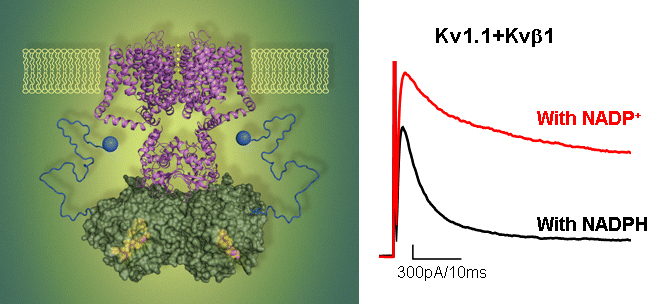
Modulation of Kv1 channels by cellular redox state and small-molecule compounds. The Shaker family voltage-dependent potassium channels (Kv1) are expressed in a wide variety of cells and essential for cellular excitability. In humans, loss-of-function mutations of Kv1 channels lead to hyperexcitability and are directly linked to episodic ataxia and atrial fibrillation. In a cell, Kv1 channels assemble with cytosolic beta subunit (Kvß) to form a stable complex. We have found that Kvß is a functional aldo-keto reductase, an enzyme that uses NADPH as cofactor to carry out a redox reaction. We have also found that Kvß’s enzymatic function is coupled to channel function: oxidation of Kvß-bound NADPH induces a large increase in channel current. Coupling between NADPH oxidation and channel activity provides a molecular basis for how excitability of a cell may be regulated by changes in cellular redox state or oxygen levels. Furthermore, that a small structural change on Kvß, for example, conversion of NADPH-to-NADP+, induces a large change in channel current, suggests that small-molecule compounds that bind to Kvß could regulate channel activity if the binding causes a structural change. The advantage of obtaining compounds that target Kvß is that these compounds will be specific to only the Kv1 channels because of the exclusive assembly between the two. There are two lines of research currently ongoing. 1. To understand mechanism of Kv1 channel modulation by cellular redox states 2. To achieve pharmacological control of Kv1 channel by targeting Kvß
Structural and functional studies of integral membrane proteins Membrane proteins make up about ~30% of all proteins in our bodies, represent ~60% of drug targets, but constitute only 1% of all known structures. Atomic level structures of membrane proteins are essential for understanding mechanisms and obtaining small-molecule modulators. As part of the New York Consortium of Membrane Protein Structures (www.nycomps.org), we use a high throughput approach to clone and express membrane proteins for structural studies. Once a high resolution structure is obtained, then functional studies are initiated to test structure-based hypotheses.
Right panel: Oxidation of the NADPH suppresses inactivation and increases channel current.
Selected Publications Cao, Y., Jin, X., Levin, E., Huang, H., Zong, Y., Quick, M., Weng, J., Pan, Y., Love, J., Punta, M., Rost, B., Hendrickson, W., Javitch, J., & Zhou, M. Crystal structure of a phosphorylation-coupled saccharide transporter. Nature, May 5, 2011; Vol 473 (7345):50-54. Pan, Y., Weng, J., Levin, E.J., & Zhou, M. Oxidation of NADPH on Kvβ1 inhibits ball-and-chain type inactivation by restraining the chain. Proc. Natl. Acad. Sci. U.S.A. April 5, 2011; Vol. 108 (14):5885-5890 Cao, Y., Jin, X., Huang, H., Derebe, M., Levin, E., Kabaleeswaran, V., Pan, Y., Punta, M., Love, J., Weng, J., Quick, M., Ye, S., Kloss, B., Bruni, R., Martinez-Hackert, E., Hendrickson, W., Rost, B., Javitch, J., Rajashankar, K., Jiang, Y., & Zhou, M. Crystal structure of a potassium ion transporter, TrkH. Nature, March 21, 2011; Vol 471 (7338):336-340. Levin, E., Quick, M., Zhou, M. Crystal structure of a bacterial homologue of the kidney urea transporter. Nature, December 10, 2009, Vol 462 (7274): 757-761. Pan, Y, Weng, J., Kabaleeswaran, V., Li, H., Cao, Y., Bhosle, R., Zhou, M. Cortisone dissociates Shaker family K+ channels from their Kvß subunits. Nature Chemical Biology; November 2008; Vol 4 (11): 708-714. Pan, Y., Weng, J., Cao, Y., Bhosle, R., Zhou, M. Functional coupling between the Kv1.1 channel and an aldo-keto reductase Kvß1. Journal of Biological Chemistry; March 28th, 2008; Vol. 283 (13): 8634-8642. Weng, J., Cao, Y., Moss, N., Zhou, M. Modulation of voltage-dependent Shaker family K channel by an aldo-keto reductase. Journal of Biological Chemistry; June 2nd, 2006; Vol 281 (22): 15194-15200. Lockless, SW, Zhou, M., Mackinnon, R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biology; May 1st, 2007; Vol 5 (5): e121, 1079-1088. Zhou, M., MacKinnon, R. A mutant KcsA K+ channel with altered conduction properties and selectivity filter ion distribution. Journal of Molecular Biology; May 7th, 2004; Vol 338 (4): 839-846. Zhou, M., Morais-Cabral, J., Mann, S., MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine blockers. Nature; June 7th, 2001; Vol 411 (6838): 657-661. Gulbis, J*, Zhou, M*, Mann, S, MacKinnon, R. Structure of the cytoplasmic ß subunit-T1 assembly of voltage-dependent K channels. Science; July 7th, 2000; Vol 289 (5476): 123-127. (* equal contribution) Grosman, C., Zhou, M., Auerbach, A.. Mapping the conformational wave of acetylcholine receptor channel gating. Nature; February 17th, 2000; Vol 403 (6771): 773-776. Zhou, M., Engel, A. G., Auerbach A.. Serum choline activates mutant acetylcholine receptors that cause slow channel congenital myasthenic syndromes. Proc. Natl. Acad. Sci. U S A. August 31st, 1999; Vol 96 (18):10466-10471.
|